Tập tin:Plant immunology.jpg
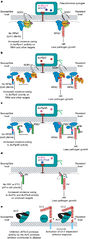
Plant immune system activation by pathogen effectors that generate modified self molecular patterns.
a, Arabidopsis RPM1 is a peripheral plasma membrane NB-LRR protein. It is activated by either the AvrRpm1 or the AvrB effector proteins. AvrRpm1 enhances the virulence of some P. syringae strains on Arabidopsis as does AvrB on soybeans. AvrRpm1 and AvrB are modified by eukaryote-specific acylation once delivered into the cell by the type III secretion system (red syringe) and are thus targeted to the plasma membrane. The biochemical functions of AvrRpm1 and AvrB are unknown, although they target RIN4, which becomes phosphorylated (+P), and activate RPM1, as detailed in the text. In the absence of RPM1, AvrRpm1 and AvrB presumably act on RIN4 and other targets to contribute to virulence. Light blue eggs in this and subsequent panels represent as yet unknown proteins. b, RPS2 is an NB-LRR protein that resides at the plasma membrane. It is activated by the AvrRpt2 cysteine protease type III effector from P. syringae. Auto-processing of AvrRpt2 by a host cyclophilin reveals a consensus, but unconfirmed, myristoylation site at the new amino terminus, suggesting that it might also be localized to the host plasma membrane. AvrRpt2 is the third effector that targets RIN4. Cleavage of RIN4 by AvrRpt2 leads to RPS2-mediated ETI. In the absence of RPS2, AvrRpt2 presumably cleaves RIN4 and other targets as part of its virulence function. c, RPS5 is an Arabidopsis NB-LRR protein localized to a membrane fraction, probably via acylation. RPS5 is NDR1-independent. It is activated by the AvrPphB cysteine protease effector from P. syringae98. AvrPphB is cleaved, acylated and delivered to the host plasma membrane. Activated AvrPphB cleaves the Arabidopsis PBS1 serine-threonine protein kinase, leading to RPS5 activation. The catalytic activity of cleaved PBS1 is required for RPS5 activation, suggesting that this 'modified-self' fragment retains its enzymatic activity as part of the RPS5 activation mechanism98. To date, no function has been ascribed to PBS1 in the absence of RPS5. d, Pto is a tomato serine-threonine protein kinase. Pto is polymorphic and hence satisfies the genetic criteria for the definition of a disease resistance protein. Pto activity requires the NB-LRR protein Prf, and the proteins form a molecular complex99. Prf is monomorphic, at least in the tomato species analysed to date. Pto is the direct target of two unrelated P. syringae effectors, AvrPto and AvrPtoB, each of which contributes to pathogen virulence in pto mutants100. It is thus likely that Prf guards Pto (refs 101, 103). The Pto kinase is apparently not required for PTI, though there may be redundancy in its function because it is a member of a gene family. e, The transmembrane RLP Cf-2 guards the extracellular cysteine protease Rcr3. Cf-2 recognizes the C. fulvum extracellular effector Avr2, which encodes a cysteine protease inhibitor. Avr2 binds and inhibits the tomato Rcr3 cysteine protease. Mutations in Rcr3 result in the specific loss of Cf-2-dependent recognition of Avr2. Hence, Cf-2 seems to monitor the state of Rcr3, and activates defence if Rcr3 is inhibited by Avr2 (ref. 104).
http://www.nature.com/nature/journal/v444/n7117/full/nature05286.html
Lịch sử tập tin
Nhấn vào một ngày/giờ để xem nội dung tập tin tại thời điểm đó.
| Ngày/Giờ | Hình nhỏ | Kích cỡ | Thành viên | Miêu tả | |
|---|---|---|---|---|---|
| hiện | 13:24, 25/11/2006 | 800×2.176 (308 kB) | Cao Xuân Hiếu (Thảo luận | đóng góp) | Plant immune system activation by pathogen effectors that generate modified self molecular patterns. a, Arabidopsis RPM1 is a peripheral plasma membrane NB-LRR protein. It is activated by either the AvrRpm1 or the AvrB effector proteins. AvrRpm1 enhances |
- Bạn không có thể ghi đè lên tập tin này.
Các trang sử dụng tập tin
Trang sau có liên kết đến tập tin này:

